SKIN-CAD – Biocom Systems, Inc.
Biocom Systems is a developer of SKIN-CAD, the only simulation software for transdermal and topical drug delivery.
Simulator for Skin Pharmacokinetics SKIN-CAD® Version 6.1
Please visit this page on SKIN-CAD users and publications.
![]() SKIN-CAD brochure English version (SKIN-CAD610E2.pdf)
SKIN-CAD brochure English version (SKIN-CAD610E2.pdf)
SKIN-CAD is…
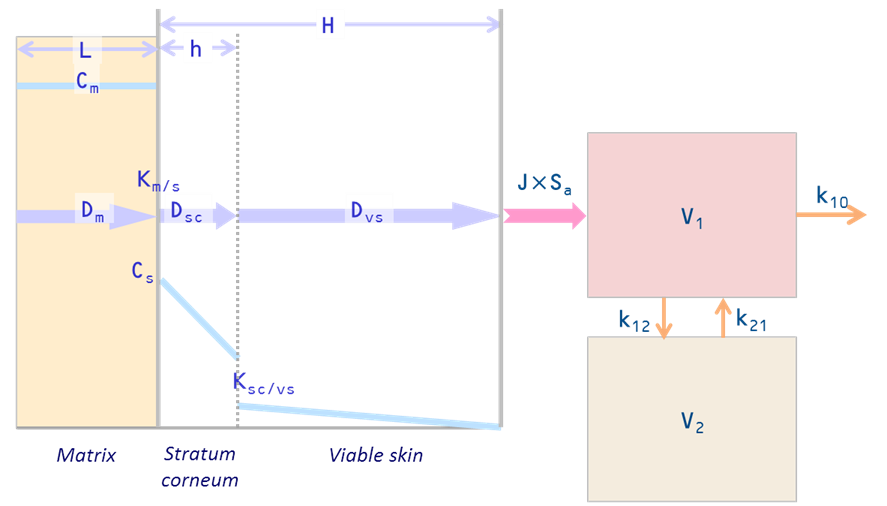
A general simulation software for the skin and body pharmacokinetics following transdermal and topical drug delivery. This software predicts dynamic profiles of skin permeation and distribution and blood concentration under various modes of transdermal delivery. This also analyzes the effects of metabolism and binding in the skin, dermal blood uptake, iontophoresis, and so on.
Features of Version 6.1
- Compatible with Windows® Vista, 7, 8, 8.1
- Compatible with 64-bit PC & OS
- Resolved “dot-commma” problem of decimal point
- Updated literature data on “skin thickness”, “skin permeation” and “body pharmacokinetics”
- Some improved functions
Capability
SKIN-CAD can optimize the design of transdermal drug delivery systems (prediction of clinical performance, setup of administration schedule) as well as evaluation of the effects of various factors on skin permeation and blood concentration profiles. With the model parameters determined from in vitro or in vivo experiments or evaluated from correlation equations, SKIN-CAD can quickly provide you with graphical images; blood concentration – time profiles for systemic application, drug distribution in the skin for topical application, skin permeability of skin-care products.
SKIN-CAD provides in vivo/in vitro/in silico evaluation of transdermal drug delivery systems.
Price Annual license system. Please contact us if you hope a quotation.
System requirements
1 (one) license-1 (one) CPU, stand-alone and user istallation
| OS | Windows Vista, 7, 8, 8.1 including 64-bit edition |
| CPU | Intel Pentium 133MHz processor (much faster recommended) |
| Display | 800 x 600 screen resolution (1024 x 768 or higher recommended) |
| Hard disk | 20MB of available space |
| Memory | 64MB of RAM (128MB or larger recommended) |
| CD-ROM drive | Required for installation |
| Browser | Required for online help (Firefox, Internet Explorer, etc.) |
Partnership
| Supervisor | Kakuji Tojo, Ph.D., Professor Emeritus, Kyushu Institute of Technology |
| Agents | (Japan) INFOCOM CORPORATION (Healthcare Service Division) FUJITSU KYUSHU SYSTEMS LIMITED (FJQS) (Life Innovation Division) (EU) FQS Poland Sp. z o.o. (subsidiary of Fujitsu Kyushu Systems Limited (FJQS)) |
Development history
| June, 2014 | Version 6.1 release |
| July, 2009 | Version 6.0 release |
| Dec., 2008 | Version 5.2 release |
| May, 2007 | Version 5.1 release |
| June, 2006 | Professional Edition Version 5.0 release |
| Dec., 2005 | Professional Edition Version 4.1 release |
| June, 2004 | Professional Edition Version 4.0 release |
| May, 2003 | Professional Edition Version 3.0 release |
| Apr., 2002 | Professional Edition Version 2.0 release |
| Aug., 2001 | Foundation of Biocom Systems, Inc. |
| Apr., 2001 | Professional Edition Version 1.1 release |
| Sep., 2000 | Professional Edition Version 1.0 release |
| May, 1999 | Start of developing SKIN-CAD for Windows |
SKIN-CAD Features
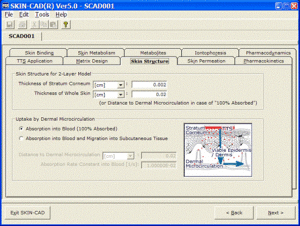
Input parameters
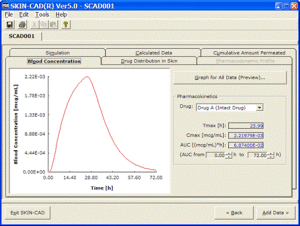
Output data
Cumulative Amount of Drug Permeated Across Skin
Blood Concentration
Distribution of Drug Concentration in Skin
Pharmacodynamic Profile
Version 6.0 features
- Addition of in vitro release data analysis
- Addition of Freundlich-type binding scheme to skin permeation model
- Addition of indirect response model to PK-PD model
- Addition of automatic searching function of steady-state profile to in vitro skin permeation data analysis
- Improvement of GUI
- Improvement of deconvolution program for blood concentration data analysis
- Addition of literature data to parameters list
Version 5.2 features
- Addition of literature database: Diffusion Coefficient in Polymer
- Addition of literature database: Skin Thickness of Human and Animals
- Addition of literature database: Diffusion and Partition Coefficients in Skin of human and animals
- Addition of literature database: Body Pharmacokinetic Parameters following intravenous administration in human
Version 5.1 features
- Vehicle compartment model: addition of simulation model: Vehicle Compartment + skin diffusion (2-layer or 1-layer) model; this feature provides simple simulation in the case of “solution” donor phase (e.g. liquid formulation, in vitro permeation system…)
- Dissolution-controlled model: modification of simulation model: Dissolution-Controlled Model added to drug-dispersed matrix model; in this model, dissolution-controlled scheme can be considered as well as diffusion-controlled step for drug release from matrix system
- PK curve fitting: modification of [Pharmacokinetics] curve-fitting function; this feature provides curve-fitting functions for PK compartment models, which are available for Continuous Intravenous Infusion and Oral Administration as well as intravenous bolus injection
- Deconvolution analysis: addition of Deconvolution Method; blood concentration data can be analyzed by deconvolution method together with i.v. bolus data, and fractional absorption can be estimated
- Improvement of graph drawing and printing
Version 5.0 features
- Curve fitting function to compartment model: curve fitting (non-linear regression analysis) function was added in order to calculate pharmacokinetic parameters. It is applicable to 1-, 2- and 3-comaprtment models from blood concentration data following intravenous administration
- Curve fitting function to Fick’s second law: curve fitting (non-linear regression analysis) function was added in order to calculate diffusion and partition coefficients in the skin. It is applicable to Fick’s second law from drug distribution data in the stratum corneum obtained by tape-stripping technique
- Search function of in vitro steady-state permeation: liniear regression program was added in order to determine the steady state for in vitro skin permeation profiles. This function makes it easy to search the steady-state profile, to evaluate permeation flux and time lag, and to calculate diffusion and partition parameters in the skin
- Flexible settings of administration schedule (application period and system size) for multiple dose
- Calculation of Tmax, Cmax and AUC for multiple administration
Version 4.1 features
- Diffusional release model of dispersed drug from matrix is added to skin permeation model
Version 4.0 features
- Pharmacodynamic (PD) analysis is possible and pharmacokinetic (PK) analysis for total body is available for 1-, 2- and 3-compartmental model
- User interface was also upgraded to be easy-to-use
“SKIN-CAD” is a registered trademark in Japan.